From: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7685956/
Eur J Pharmacol. 2021 Feb 5; 892: 173751.
Published online 2020 Nov 25. doi: 10.1016/j.ejphar.2020.173751
PMCID: PMC7685956
PMID: 33245898
COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies
Kajal Rawat, Puja Kumari, and Lekha Saha∗
Author information Article notes Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Abstract
Graphical abstract
1. Introduction
By the end of year 2019, Wuhan city of China had witnessed several cases of pneumonia like conditions, which later on found to be caused by 2019-novel coronavirus (2019-nCoV) (Zhou et al., 2020b). Later, on February 11, 2020, the novel coronavirus was termed as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the international virus classification commission and on the same day the disease caused by SARS-CoV-2 was termed as Coronavirus Disease 2019 (COVID-19) by World Health Organization (WHO) (Li et al., 2020a, Li et al., 2020b). WHO had declared COVID-19 as a global health emergency on January 30, 2020 and characterized it pandemic on 11 March 2020. The case fatality ratio (CFR, defined as the proportion of individuals diagnosed with a disease who die from that disease) was reported as 1–3% and it was shown to be higher in elderly patients especially men (Spychalski et al., 2020). CFR is therefore a measure of severity of infection among detected cases, which is found to be varying among the WHO defined region (summarized in Table 1 ). The probable reason could be the region specific immune response of individuals towards the infection. Since the southeast Asian countries are well immune to SARS and MERS infection, the fatality ratio is comparatively lesser than those in WHO defined American and European region. This potentially fatal virus is known to transmit quickly and have spread across 216 countries and territories affecting more than 45 million people worldwide (WHO, 2020). This came within the 11 months after the first case have reported in Wuhan. Given the severity of the disease, there is an urgent need of therapeutics or vaccines to treat or prevent the spread of SARS-CoV-2.
Table 1
Case fatality rate across the world as per WHO.
|
WHO Region |
Confirmed Cases |
Deaths |
CFR |
|
Americas |
19,737,794 |
625,973 |
3.17% |
|
Europe |
9,664,042 |
270,972 |
2.8% |
|
South East Asia |
8,969,707 |
140,827 |
1.5% |
|
Eastern Mediterranean |
2,955,552 |
75,133 |
2.5% |
|
Africa |
1,298,315 |
29,277 |
2.2% |
|
Western Pacific |
715,300 |
15,314 |
2.1% |
Currently, no potential drugs are available to treat COVID-19. Only strategies followed worldwide to combat COVID-19 is to relieve the patient's symptoms. There are many therapeutic approaches being tried all over the world to tackle SARS-CoV-2 infections. One approach is to understand the structure, life cycle and pathogenesis of the virus, which ultimately facilitates a deeper understanding of various targets to be approached for devising a specific drug for SARS-CoV-2. Another approach like repurposing of existing drugs, such as those for influenza, hepatitis B, hepatitis C, and filoviruses, was considered to allow more rapid development (Li and De Clercq, 2020). Various therapeutic treatments like antiviral drugs (nucleotide analogue) (Cao et al., 2020; Holshue et al., 2020; Wang et al., 2020), antimalarial drugs (those inhibits viral cell entry and its replication) (Gao et al., 2020; Gautret et al., 2020), immunomodulators (Bian et al., 2020) and cell and plasma-based therapy (Leng et al., 2020) are currently being used worldwide. There are number of clinical trials ongoing all over the world in search of a specific treatment for COVID-19. Despite the various approaches there are number of cases where patients once declared clinically recovered from COVID-19 have suffered reinfection (An et al., 2020; Xing et al., 2020; Yuan et al., 2020) and these shortcomings of current approaches highlight the necessity of vaccines against SARS-CoV-2. For the development of a vaccine against SARS-CoV-2, numbers of approaches are being tried all over the world like targeting S protein of the virus and generating antibodies against this. Besides this various other vaccines are designed against the virus, including RNA and DNA based vaccines and these are described further in details.
Scientists and researchers all over the world are trying to understand this novel virus, SARS-CoV-2, to develop specific treatments for controlling and preventing this potentially fatal disease. Immune system plays pivotal role in the pathogenesis of SARS-CoV-2 infection, and to develop understanding of the immune response and the underlying mechanism is very important in developing an effective vaccine. In this review, we discuss different targets that can be approached for vaccine design and development against SARS-CoV-2 infections. Also, we discuss different platforms that are currently being used all over the world for the development of vaccine candidates.
2. Key features of human coronaviruses
Coronaviruses are positive-sense single-stranded viruses with RNA as genetic material and they are named so because of their crown like shape obtained by the presence of surface glycoproteins (Cui et al., 2019). These viruses mainly cause infection in birds and mammals. In humans, these generally cause mild respiratory infections with common cold like symptoms. However, in the past two decades, three of the human coronavirus of zoonotic origin including SARS-CoV, MERS-CoV and SARS-CoV-2 have resulted in lethal endemics in 2002, 2012 and 2019 respectively. All of these three viruses belong to the genus Beta coronavirus within the family Coronaviridae (Ahmed et al., 2020). The comparative characteristics are tabulated in Table 2 . Studies have shown that SARS-CoV-2 is most likely originated in bats but some intermediate host might be there responsible for amplifying the virus (Zhou et al., 2020b). Another study has suggested pangolins as a potential amplifying host for SARS-CoV-2 (Zhang et al., 2020a, 2020b). The similarity between existing SARS-CoV and novel SARS-CoV-2 helped in revealing the entry mechanism of virus into host. Studies have shown that just like SARS-CoV, SARS-CoV-2 used angiotensin-converting enzyme-2 (ACE2) receptor for entering into hosts ranging from bats to humans including all the animals that express ACE2 receptor (Letko et al., 2020; Wan et al., 2020). The swift genomic sequencing of SARS-CoV-2 remains helpful in determining its genetic similarity with MERS-CoV and SARS-CoV (Lu et al., 2020). This has been helpful in identifying the genomic regions of interest that can be targeted therapeutically for prevention as well as treatment.
Table 2
Comparative characteristics of zoonotic beta cornoviruses lethal to humans.
|
Characteristics |
SARS-CoV-2 |
MERS-CoV |
SARS-CoV |
|
Date first detected |
December 2019 |
June 2012 |
November 2002 |
|
Potential Reservoir Host |
Bat |
Dromedary Camels (WHO) |
Bat |
|
Possible Intermediate host |
Pangolin |
Camel |
Palm civets |
|
Target cellular receptor |
Angiotensin converting enzyme 2 (ACE2) |
Dipeptidyl peptidase 4 (DPP4) |
Angiotensin converting enzyme 2 (ACE2) |
|
Incubation Period |
2–14 days |
2–10 days |
2–10 days |
|
Speed of spread |
High |
Low |
Moderate |
3. SARS-CoV-2 structure and targets for vaccine development
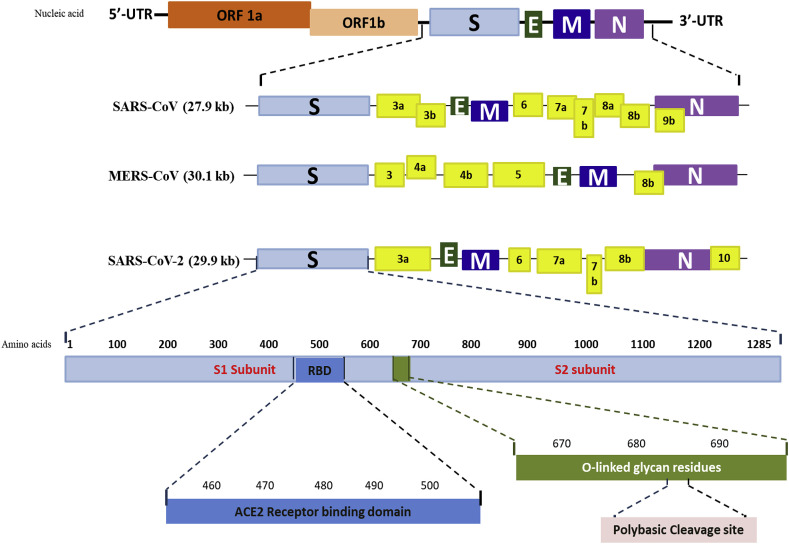
SARS-CoV-2 is a spherical shaped virus with genome size of 30 kb. The two third of the genome of SARS-CoV-2 shows expression of the replicase and the other part of genome codes for structural and accessory proteins (Adnan et al., 2020). The four types of structural proteins are spike protein (S), envelope protein (E), membrane glycoprotein (M) and nucleocapsid protein (N). The genetic sequence of SARS-CoV-2 was published on 11 January 2020 by Wu et al. (2020c). Studies have shown approximately 80% similarity in the RNA genome of SARS-CoV-2 and SARS-CoV yet notable variations are reported in the genome of both these viruses as well as with MERS-CoV genome as shown in Fig. 1 A. These differences include the absence of 8a protein and difference in the number of amino acids that codes for 8b and 3c protein in SARS-CoV-2 (Wu et al., 2020a) (Fig. 1A). It is also reported that genome of SARS-CoV-2 has undergone genetic recombination with SARS-related CoV and it might be a combination of SARS-CoV of bat and unknown beta-CoV (Zhang et al., 2020). This new strain of SARS-CoV-2 can easily recognize the evolving human ACE2 receptor, and therefore its S protein possesses efficient transduction efficiency resulting in the fast transmission of SARS-CoV-2 in humans (Walls et al., 2020). The S protein comprises of two functional subunits S1 and S2 subunits, S1 subunit being responsible for interaction with the host's ACE2 receptor through Receptor Binding Domain (RBD) and S2 subunit responsible for the fusion of viral and cellular membranes. The S protein also contains a functional polybasic (furin) cleavage site at the S1–S2 subunit boundary which led to the acquisition of three O-linked glycans around the site (Fig. 1A). Both the polybasic cleavage site and the three adjacent predicted O-linked glycans are unique to SARS-CoV-2 and were not previously seen in lineage betacoronaviruses (Andersen et al., 2020; Hu et al., 2020).
Schematic diagram of genomic organization or open-reading frames (ORFs) of SARS-CoV, MERS-CoV and SARS-CoV-2. Variations in accessory proteins (yellow) in all the three strains of betacoronvirus organized within structural proteins, Spike (light blue, S), envelope (green, E), membrane (dark blue, M), nucleocapsid (purple, N) and non-structural proteins expressed in ORF 1a (brown) and ORF 1b (peach) are indicated. Untranslated regions at the N and C-terminals are represented respectively as 5′-UTR and 3′-UTR; kb indicates kilo base pairs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
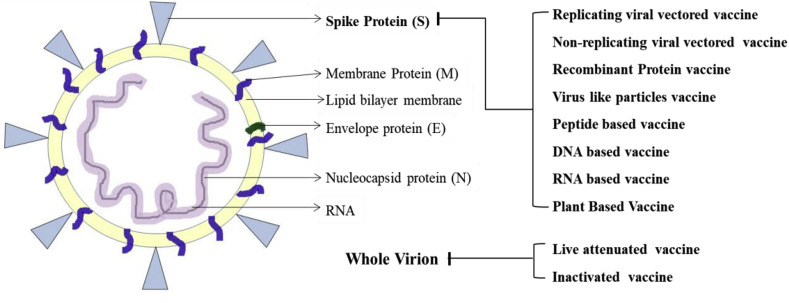
The continuous evolution of the novel coronavirus poses its ability to quickly transmit among population (Zhang et al., 2020). The similarity between the two viruses can be helpful in vaccine development against SARS-CoV-2 with the help of previously researched protective immune response against SARS-CoV. Studies have demonstrated that S protein is a prime target for the designing and developing vaccines (Ahmed et al., 2020). It is responsible for the attachment of virus to the host's cell surface receptor (most likely ACE2). Just as in SARS-CoV, antibodies targeting the S protein of SARS-CoV-2 can interfere with the virus resulting in neutralization of the infection caused by the virus (Liu et al., 2017). Hence, many vaccines developing platforms containing target antigen commonly target the S protein (Fig. 1 B).
Schematic structure of SARS-CoV-2 with its key structural proteins as target antigens for various vaccines production platforms. S protein is the major target antigen for most of the platforms except the conventional ones (Live attenuated and Inactivated vaccines) where the whole virion or the subunit of it is used to develop vaccines.
4. COVID-19 immunopathology and vaccine designing
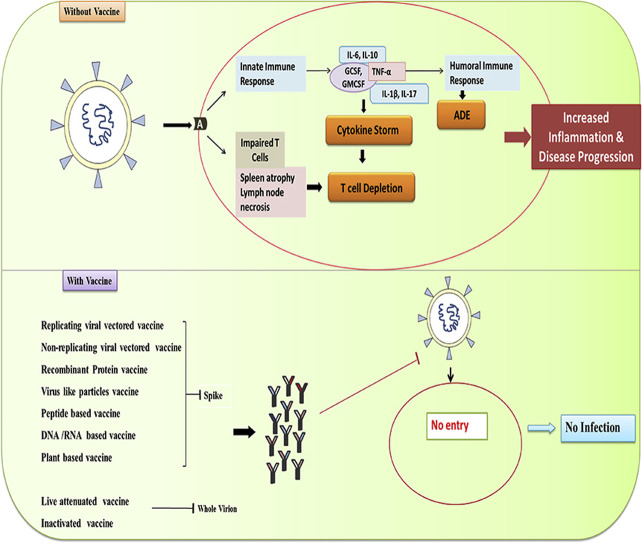
The immunopathology of COVID-19 explains the reaction of immune system against novel coronavirus infection. SARS-CoV-2 attacks hosts' immune system leading to an uncontrolled inflammatory responses, lymphocytpenia, high cytokine levels and increased antibodies. The most prominent immuno invasion processes observed in patients were:
· Depletion of lymphocytes,
· increased neutrophils,
· cytokine storm and
· antibody dependent enhancement (Yang et al., 2020a).
The SARS-CoV-2 virion
1. attacks ACE2 receptors on type 2 alveolar cells and
2. release(s) the viral genome (RNA) into the cytoplasm of host cell,
3. the RNA further gets translated into structural proteins and
4. then begins to replicate (Perlman and Netland, 2009).
5. The viral particles subsequently undergo transformation in the endoplasmic reticulum-Golgi intermediate compartment and
6. released entrapped into vesicles,
7. which on fusion with plasma membrane release the viral particles (De Wit et al., 2016).
Simultaneously, while virus enters the cells, a part of it as antigen is presented on the surface of antigen presentation cells (APC), which is crucial for the body's immune response. Antigen presentation on B and T cells ultimately leads to the production of the typical pattern of IgM and IgG antibodies. Researchers have found that these IgM antibodies lasts until week 12, while the IgG antibodies remain long lasting and can provide prolonged protection (Li et al., 2003). These type of virus-specific neutralizing IgG antibodies play major role by regulating the infection at later phase and prevents reinfection in the future (De Wit et al., 2016). In general, T cells are the major immune cells those fight against viral infections in body either by production of virus specific antibodies (CD4+ helper type T cells mediated response) or by killing the virus-infected cells (CD8+ cytotoxic type T cells mediated response) (Mubarak et al., 2019). However, the report of COVID-19 patients shows marked reduction in CD4+ and CD8+ T cells population, whereas the high proportions of HLA-DR (CD4 3.47%) and CD38 (CD8 39.4%) double positive fractions indicates the hyperactivated status of T cells imparting severe immune injury (Xu et al., 2020). There could be the several possible mechanisms involved in the depletion and dysfunctioning of T lymphocytes induced by SARS-CoV-2 such as,
(1) SARS-CoV-2 can directly infect T cells and macrophages by binding to the surface markers (CD 26 and CD 147) (García, 2020) or the ACE-2 receptors present on their surface (Dandekar and Perlman, 2005) as well as SARS-CoV-2 induce spleen atrophy and lymph node necrosis thereby destroying lymphatic organs (Cao, 2020; Li et al., 2020a, Li et al., 2020b; Tan et al., 2020);
(2) another mechanism is cytokine storm induced by SARS-CoV-2, the increased level of cytokines like TNFα, IL-6, and IL-10 is inversely correlated with the decreased T cell population (Moon, 2020). The lymphocytopenia occurring in the patients further explains the increased neutrophil count in patients, since the depletion of T cells make patients more susceptible to microbial infections and thereby promoting the recruitment and activation of neutrophils in patients' blood (Deshmukh et al., 2014).
The clinical outcomes and associated manifestations are briefly summarized in Table 3 .
Table 3
Symptoms and Clinical outcomes of SARS-CoV-2 induced immunopathology in humans.
|
Organ System |
Clinical Outcomes |
Clinical Manifestations |
References |
|
Vascular System |
Cytokine storm |
Elevated IL-6, TNFα, IL-1β |
(Huang et al., 2020; Rodrigues et al., 2020; Zhou et al., 2020a, Zhou et al., 2020b, Zhou et al., 2020c) |
|
Lymophocyte count |
Elevated T helper 17 cells (TH17), plasma cells, CD8+ T cell activity, and decreased regulatory T cells |
||
|
Thrombocytopenia associated mortality |
Reduced platelet to lymphocyte ratio |
||
|
ARDS |
Elevated levels of ferritin |
(Wu et al., 2020a, Wu et al., 2020b, Wu et al., 2020c; Zhou et al., 2020a, Zhou et al., 2020b, Zhou et al., 2020c) |
|
|
Vasculitis and Vascular Dysfunction |
Elevated VEGF, IL-10, IL-8 |
(Becker, 2020; Huang et al., 2020; Zhou et al., 2020a, Zhou et al., 2020b, Zhou et al., 2020c) |
|
|
Lungs |
Pneumonia with ARDS and dyspnea |
Elevated IL-6, TNFα, IL-1β, IL-10, IL-8 |
|
|
Kidneys |
Proteinuria and hematuria |
Elevated urea and creatine levels |
(Cheng et al., 2020; Su et al., 2020; Yang et al., 2020a, Yang et al., 2020b) |
|
Liver |
Steatosis and abnormal Liver function |
Elevated AST, ALT, CRP, albumin levels |
(Ling, 2020; Zhou et al., 2020a, Zhou et al., 2020b, Zhou et al., 2020c) |
|
Heart |
Acute myocardial injury and chronic CVS damage |
Elevated CK and LDH levels |
(Huang et al., 2020; Yang et al., 2020a, Yang et al., 2020b) |
|
Intestine |
Microbial infection, diarrhea, severe acute ulcerative colitis |
Reduced T cell and NK cell count (Lymphopenia) |
(Carvalho et al., 2020; D'Amico et al., 2020; Yang et al., 2020a, Yang et al., 2020b) |
|
Brain |
Encephalopathy, headache, ischemic stroke |
Elevated CRP, D-dimer, ferritin levels |
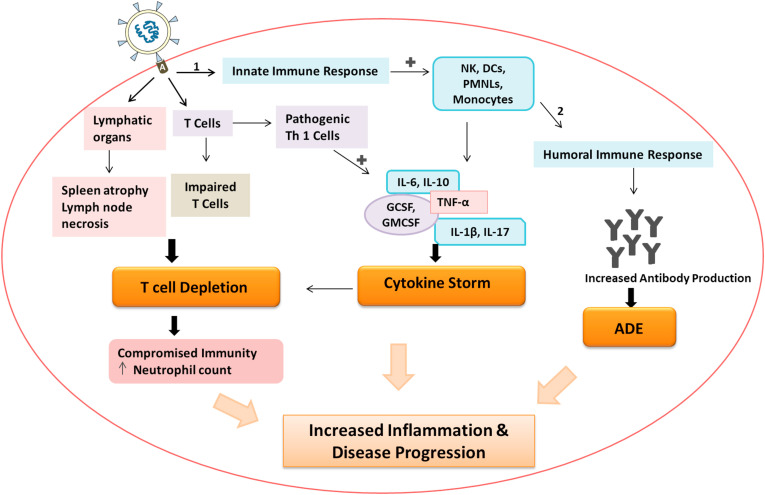
Apart from lymphocytopenia, cytokine storm is another major clinical outcome observed in patients with severe COVID-19. SARS-CoV-2 after invading the host, activates host's immune system first inducing innate immune response and then subsequently generating the adaptive immunity. The innate response is marked by the production of cytokines specifically Type-I and –III antiviral Interferons and different chemokines (Hur, 2019). Chemokines induce infiltration of more innate cells like monocytes, Natural Killer cells, dendritic cells, polymorphonuclear leukocytes, which produces more chemokines (García, 2020). On the other hand direct activation of T cells by the SARS-CoV-2 infection leads to the activation of pathogenic T helper 1 (Th1) cells that secrete GM-CSF and produce inflammatory cytokine IL-17 which recruits macrophages and neutrophils to the site of infection and stimulates IL-1β and IL-6 cytokine cascades, where IL-6 plays the major role in development of cytokine storm in COVID-19 patients (Wu and Yang, 2020; Zhou et al., 2020c). T cells ultimately led to the activation of B lymphocytes which induce humoral response by producing neutralizing antibodies. COVID-19 patients were found to have poor outcome even after antibody upregulation, this can be explained by the phenomenon Antibody Dependent Enhancement (ADE) (Ulrich et al., 2020). Various infections like Ebola, Dengue, MERS have shown the antibody dependent enhancement of viral infection where it was observed that the viral entry and replication is being enhanced by sub-neutralizing antibodies (Cao, 2020; Katzelnick et al., 2017; Roncati et al., 2020). However, the ADE mediated inflammatory response and its association with disease severity and progression requires further investigation (Yang et al., 2020a). The interconnection between all these processes collectively giving rise to lung injury and disease progression is briefly schematized in Fig. 2 .
Schematized representation of immunopathogenesis of SARS-CoV-2. SARS-CoV-2 acting on ACE-2 receptor (A) giving rise to the cascade of pathological mechanisms including, Activating innate line of defense (1) and subsequently giving rise to Humoral response (2), leading to Antibody dependent enhancement (ADE), T cell depletion, Lymphatic organs damage and Cytokine storm.
Studies on SARS-CoV patients have shown the presence of memory T cells after the four years from recovery and those memory T cells remain able to respond against the S protein of SARS-Cov (Fan et al., 2009; Tang et al., 2011). The similar results were reported in MERS coronavirus infection in mice (Zhao et al., 2014). The aftermath, strongly indicates the importance of T cells in controlling the previously existing SARS and MERS coronavirus infections; hence there is high possibility that it can also provide protection against novel coronavirus, SARS-CoV-2 infection. The epitopes identified for SARS and MERS coronaviruses mostly concentrate on structural proteins especially S protein, hence mapping these epitopes against SARS-CoV-2 can be beneficial in designing the vaccine against all three human coronaviruses in the future (Mubarak et al., 2019).
4.1. Role of bioinformatics in vaccine designing-
The remarkable scientific and technological advancements since last century have played a major role in the improvement of vaccines’ potency as well as in speeding up their production. The fusion of computational technologies with the recombinant DNA technology, the possibility of accelerated and massive sequencing of complete genomes and the fast growth of biological and genomic information in database banks have made this possible (He et al., 2010; María et al., 2017). Reveres vaccinology, where one could identify the structure of the bacteria, virus, cancer cell or any pathogen or allergen capable of inducing immune response using the bioinformatics tools, possess various advantages over traditional vaccine production methodology like reduced time and cost of vaccine development (Rappuoli, 2000; Seib et al., 2012). A wide variety of softwares are available providing various functions ranging from genomic sequencing of an organism to finding out the possible immunogenic response associated with different proteins, thereby easing the vaccine development process. Also there are websites that with the help of 3D structure of protein, can predict conformational epitopes for B cells (María et al., 2017). NIAID Virus Pathogen Database and Analysis Resource (ViPR) and Immune Epitope Database (IEDB) helped in comparing epitopes of SARS COV 1 and SARS COV 2 which further found helpful in epitope mapping and targeting SARS-CoV-2 spike protein in order to develop various vaccines against the infection (Ahmed et al., 2020).
5. SARS-CoV-2 vaccine production platforms
The rapid transmission of SAR-CoV-2 infection across the world ignited extensive efforts toward the development of COVID-19 vaccines that can be used globally for ending the pandemic. The full length spike glycoprotein or RBD of virion are able prevent host and virus interaction by inducing neutralizing antibodies and hence is considered as most important vaccine target antigen (Prompetchara and Chutitorn Ketloy, 2020). Most of the COVID-19 vaccine development projects ongoing all over the world are using S protein as target antigen (Table 4 ).
Table 4
List of COVID-19 Vaccine candidates under clinical evaluation stage.
|
Vaccine Platform |
Vaccine Name |
Vaccine characteristics/Immunogen |
Stage of Developmenta |
|
Non Replicating Viral vector |
ChAdOx1-S nCoV-19 vaccine |
Adenovirus Type 5 vector that expresses S protein |
Phase 3 NCT04516746 ISRCTN89951424 NCT04540393 CTRI/2020/08/027170 |
|
Ad-5 vectored COVID-19 vaccine |
Weak recombinant adenovirus carrying S protein expression |
Phase 3 NCT04526990 NCT04540419 |
|
|
Gam-COVID-Vac |
Adeno-based vectored combined (rAd26-S + rAd5-S) expressing S protein |
Phase 3 NCT04530396 NCT04564716 |
|
|
Ad26.COV2.S |
Adenovirus vectored vaccine |
Phase 3 NCT04505722 |
|
|
GRAd-COV2 |
Replication defective Gorilla Adenovirus that encodes for SARS-COV-2 Spike protein |
Phase 1 NCT04528641 |
|
|
hAd5-S-Fusion + N-ETSD vaccine |
Human adenovirus serotype 5 (hAd5) vector with E1/E2b/E3 deletions expressing viral S fusion protein and nucleocapsid with an enhanced T-cell stimulation domain (ETSD). |
Phase 1 NCT04591717 |
|
|
Ad5-nCoV |
Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) |
Phase 1 NCT04552366 |
|
|
VXA-CoV2-1 |
Ad5-vector based vaccine expressing a SARS-CoV-2 antigen and dsRNA adjuvant given as oral tablets |
Phase 1 NCT04563702 |
|
|
MVA-SARS-2-S |
Modified Vaccinia Virus Ankara (MVA) vector expressing the SARS-CoV-2 spike protein (S) |
Phase 1 NCT04569383 |
|
|
DNA |
INO-4800 |
DNA plasmid encoding S protein with electroporation delivery mechanism |
Phase 1/2 NCT04447781 NCT04336410 |
|
AG0301-COVID19 |
DNA plasmid vaccine administered with adjuvant |
Phase 1/2 NCT04463472 NCT04527081 |
|
|
Novel Corona Virus-2019-nCov vaccine |
DNA plasmid vaccine expressing S protein |
Phase 1/2 CTRI/2020/07/026352 |
|
|
GX-19 |
DNA vaccine expressing SARS-CoV-2 S-protein antigen |
Phase 1/2 NCT04445389 |
|
|
RNA |
BNT162b1 |
Prophylactic SARS-CoV-2 mRNA enclosed into lipid nanoparticle |
Phase 3 NCT04368728 |
|
mRNA 1273 |
Novel lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine that encodes for a full-length spike (S) protein of SARS-CoV-2 |
Phase 3 NCT04470427 |
|
|
CVnCoV mRNA vaccine |
mRNA |
Phase 2 NCT04515147 |
|
|
ARCT-021 mRNA vaccine |
Self-replicating mRNA encoding SARS-Cov-2 spike protein encapsulated in lipid nanoparticle |
Phase 1/2 NCT04480957 |
|
|
LNP-nCoVsaRNA vaccine |
Purified mRNA which mimics the virus gene for a spike protein on its surface |
Phase 1 ISRCTN17072692 |
|
|
SARS-CoV-2 mRNA vaccine |
mRNA vaccine |
Phase 1 ChiCTR2000034112 ChiCTR2000039212 |
|
|
Inactivated |
Adsorbed COVID-19 (inactivated) Vaccine |
Inactivated SARS-CoV-2 with adjuvant alum |
Phase 3 NCT04456595 669/UN6.KEP/EC/2020 NCT04582344 |
|
Inactivated SARS-CoV-2 vaccine (Vero cell) |
Inactivated SARS-CoV-2 |
Phase 3 ChiCTR2000034780 ChiCTR2000039000 |
|
|
Inactivated SARS-CoV-2 vaccine (Vero cell) |
Inactivated SARS-CoV-2 |
Phase 3 ChiCTR2000034780 NCT04560881 |
|
|
Inactivated SARS-CoV-2 Vaccine |
Inactivated SARS-CoV-2 |
Phase 1/2 NCT04470609 |
|
|
QazCovid-in® - COVID-19 inactivated vaccine |
Inactivated SARS-CoV-2 |
Phase 1/2 NCT04530357 |
|
|
BBV152 |
Whole Virion Inactivated SARS-CoV-2 |
Phase 1/2 NCT04471519 CTRI/2020/09/027674 |
|
|
Inactivated SARS-CoV-2 Vaccine (Vero Cells) |
Inactivated SARS-CoV-2 |
Phase 1 ChiCTR2000038804 |
|
|
Replicating Viral Vector |
TMV-083 |
Live-attenuated recombinant measles vaccine virus vector expressing a modified surface glycoprotein of SARS-CoV-2 |
Phase 1 NCT04497298 |
|
V590 |
Replication-competent VSV delivering the SARS-CoV-2 Spike |
Phase 1 NCT04569786 |
|
|
DelNS1-2019-nCoV-RBD-OPT1 |
Intranasal flu-based-RBD |
Phase 1 ChiCTR2000037782 |
|
|
rVSV-SARS-CoV-2-S Vaccine |
Replication-competent SARS-CoV-2 Spike protein |
Phase 1 NCT04608305 |
|
|
Protein Subunit |
SARS-CoV-2 rS |
Full length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M |
Phase 3 2020-004123-16 |
|
Recombinant new coronavirus vaccine (CHO cells) |
Adjuvanted recombinant protein (RBD-Dimer) |
Phase 2 NCT04466085 |
|
|
KBP-COVID-19 |
RBD-based |
Phase 1/2 NCT04473690 |
|
|
Recombinant S protein |
Recombinant S protein (baculovirus production) with different adjuvants |
Phase 1/2 NCT04537208 |
|
|
SCB-2019 |
Native like Trimeric subunit Spike Protein vaccine |
Phase 1 NCT04405908 |
|
|
COVAX-19 vaccine |
Recombinant spike protein with Advax™ adjuvant |
Phase 1 NCT04453852 |
|
|
MF59 adjuvanted SARS-CoV-2 Sclamp vaccine |
Molecular clamp stabilized Spike protein with MF59 adjuvant |
Phase 1 ACTRN12620000674932p ISRCTN51232965 |
|
|
MVC-COV1901 |
Spike protein with CpG 1018 and aluminum content as adjuvant |
Phase 1 NCT04487210 |
|
|
SOBERANA 01 |
RBD + Adjuvant |
Phase 1 IFV/COR/04 |
|
|
EpiVacCorona |
Peptide antigens of SARS-CoV-2 proteins, conjugated to a carrier protein and adsorbed on an aluminum-containing adjuvant (aluminum hydroxide) |
Phase 1 NCT04527575 |
|
|
Recombinant SARS-CoV-2 vaccine (Sf9 cell) |
RBD (baculovirus production expressed in Sf9 cells) |
Phase 1 ChiCTR2000037518 |
|
|
pVAC |
SARS-CoV-2 HLA-DR peptides |
Phase 1 NCT04546841 |
|
|
UB-612 |
High-precision designer S1-RBD-protein based vaccine containing a Th/CTL epitope peptide pool, that could bind to human MHC-I and MHC-II to activate T cells |
Phase 1 NCT04545749 |
|
|
Virus Like Particle |
RBD-HBsAg VLPs |
RBD antigen is conjugated to the hepatitis B surface antigen that stimulates immune system to produce anti-RBD antibodies |
Phase 1/2 ACTRN12620000817943 |
|
Coronavirus-Like Particle COVID-19 Vaccine |
Plant-derived VLP unadjuvanted or adjuvanted with either CpG 1018 or AS03 |
Phase 1 NCT04450004 |
aSource: (Cochrane, 2020; National Library of Medicine, 2020; World Health Organization, 2020).
In the last decade, there is the marked evolution of vaccine platforms including the development of nucleic acid based vaccine candidates, vectored vaccines and recombinant protein vaccines. SARS-CoV-2 vaccine candidates currently being developed worldwide and under evaluation are based on different vaccine platforms briefly detailed in Fig. 1B. Some of the platforms are already in use for different vaccines and are licensed for use by different regulatory bodies while some of them are still in trials. However, the understanding of these in the field of oncology has encouraged researchers to use next-generation approaches in order to increase the speed of development and manufacture.
5.1. Replicating and non replicating vectors
Based on their capability to replicate in the host cell, these can be replicating and non replicating recombinant viral vectors. These are evaluated as delivery of viral genome encoding the gene of interest. The longevity of the immune response generated by vaccine depends on the type of viral vector used. Most commonly used viral vector is Adenoviral (Ad) vector. The major advantage of this platform is the capability to induce both humoral and cellular immunity. Despite the complex production of viral vector based vaccines, these are known to induce strong immunological response (Geiben-Lynn et al., 2008; Rollier et al., 2011). However, sometimes these are not capable of inducing immunogenicity due to the presence of preexistent immunity (Sekaly, 2008). A recombinant replicating vaccine, Ervebo is licensed in the European Union as ebolavirus vaccine (Marzi et al., 2011). The adenoviral vector Ad5 being used for COVID-19 vaccine development is cost effective approach and already been used for Ebola virus. One of the limitations of Ad5 vectors is their association with high prevalence in the human population and therefore an additional trial using chimpanzee derived adenoviruses (ChAd) is being conducted to combat preexisting immunity. Currently, there are 9 COVID-19 vaccine candidates developed using non replicating vector platform in clinical evaluation and 19 candidates in preclinical evaluation stage. Four COVID-19 vaccine candidates developed using replicating vector platform is in clinical evaluation stage and 18 candidates are in preclinical evaluation stage (World Health Organization, 2020).
5.2. Virus-like particles (VLPs)
These are protein multimers mimicking the structure of real virus but lacking genetic material and hence are non-infectious in nature. These can self assemble with more than one type of protein forming protein chimeras known as cVLPs (Roldão et al., 2010). VLPs act by stimulating antigen presenting cells mediated activation of B- and T-cell immune responses. These are also involved in CD8+ cytotoxic T cell mediated killing of pathogenic cells. The immune system recognizes VLPs in the same way as it recognize original virus and thereby induce immune responses (Hemann et al., 2013). VLP formulations because of their poor immunogenicity require adjuvants in most of the cases. VLP-based vaccines are well established platform for prophylactic use. These are less time taking and production cost depends upon the expression system used which is comparatively low for bacterial system than the mammalian expression system. The licensed vaccines based on this platform are currently in use for human papillomavirus (HPV) (Zhao et al., 2013). Currently, there are two COVID-19 vaccine candidates developed as VLPs in clinical evaluation and 15 COVID-19 vaccine candidates in preclinical evaluation stage developed (World Health Organization, 2020).
5.3. DNA platform
DNA-based vaccines were introduced two decades ago and these are non-infectious and non-replicating. These are easy to produce within a short duration and are stable and cost-effective at the same time. These confer long term immunogenicity to the host, however these remain hopeless when used in humans due to their poor immunogenic property. Also these are easily degraded by host enzymes and there is always the risk of its integration into host DNA. While it has shown efficacy in animal models, but yet to establish the potency in human trials (Kim , 2009). Currently, there are 4 COVID-19 vaccine candidate in clinical evaluation and 14 candidates in preclinical evaluation stage developed using DNA platform (World Health Organization, 2020).
5.4. RNA platform
After the failure of DNA based vaccines, researchers established the RNA-based vaccines which have several advantages over DNA based vaccines. The mRNA is directly injected into the host's cell, which undergo translation in the cytoplasm. Currently, there are two kinds of mRNA-based vaccines established: non-amplifying mRNA based vaccines and self-amplifying mRNA based vaccines (Rodríguez-Gascón et al., 2014). The self-amplifying mRNA based vaccines (SAM) capable of being rapidly developed are potentially feasible for epidemics or disease outbreaks as observed in Shanghai, where within 7 days of H7N9 influenza outbreak the vaccine developed by SAM technology was tested in mice (Hekele et al., 2013). The SAM vaccine technology is capable of swift and cost-effective vaccines production. These can be devised for both therapeutic as well as prophylactic purpose as shown by variety of preclinical and clinical projects while the poor immunogenicity remains their biggest limitation although it can induce antigen-specific T and B cell immune response. Currently, there are 6 COVID-19 vaccine candidates in clinical evaluation and 19 candidates in preclinical evaluation stage developed using RNA platform (World Health Organization, 2020).
5.5. Recombinant protein based vaccine
Highly purified recombinant proteins from different etiologic agents are most common candidates under investigation for vaccines. Different genes from virus particles encoding the antigenic determinant have been processed (cloned, expressed in various expression system and purified) as recombinant proteins and established as vaccines. For the antigens which undergo post-translational modifications, mammalian cells expression systems can used while the bacterial expression systems provide the ease of handling and high level expression (Nascimento and Leite, 2012). It is the most common of all the platforms used for production of vaccines since it possesses several advantages such as safety profiles and cost effective production but the adjuvants are required to produce long-lasting immune response. There are several examples of recombinant protein based vaccines being used in humans like hepatitis B vaccine (HBV) (Kumar and Meldgaard, 2018). Currently, there are 13 COVID-19 vaccine candidates in clinical evaluation and 55 COVID-19 vaccine candidates in preclinical evaluation stage developed as recombinant protein subunit (World Health Organization, 2020).
5.6. Live attenuated vaccines
The most common traditional method which involves manually weakened live pathogen which is no longer able to induce infection but able to induce immune response and hence mimic features of natural infection. It is capable of inducing both humoral and cellular immune response. Live attenuated vaccines on intranasal administration induces secretion of IgA and hence provide local mucosal immunity (Barría et al., 2013). These vaccines are popular to induce strong lifelong immune responses within two doses. These are easy to produce for some viruses but challenging for complex pathogens. Most common example is Oral Polio Vaccine (Kumar and Meldgaard, 2018). Currently, there are 3 COVID-19 vaccine candidates in preclinical evaluation stage developed using this platform (World Health Organization, 2020).
5.7. Inactivated vaccines
These are produced by completely inactivating or killing the pathogen, on injecting it to the host, they primarily induce protective antibodies against epitopes on hemagglutinin glycoprotein on surface of virus. The subunit formulations that primarily consist of a part of killed pathogen in spite of the whole pathogen are used more frequently than the other formulations because potential association of whole virus formulation with increased reactogenicity. These vaccines tend to produce a weaker immune response than live attenuated vaccines, thus adjuvants are required to provide an effective immune response (Kumar and Meldgaard, 2018). Inactivated Polio Vaccine is the classical example where the whole killed pathogen is provided and Diphtheria and Tetanus vaccination is the example of subunit formulation (Nascimento and Leite, 2012). Currently, there are 7 COVID-19 vaccine candidates in clinical evaluation and 12 candidates in preclinical evaluation stage developed using this platform (World Health Organization, 2020).
5.8. Plant based vaccines
There are numerous limitations of injectable vaccines including its high cost and maintenance, transportation, and requirements of trained medical personnel, making its availability more difficult specifically in the developing countries. On contrary, plant based vaccines being capable of producing edible vaccines can be easily available globally to the needy population. Various ongoing reports have investigated the development and stability of edible vaccines (Sartaj Sohrab et al., 2017). The another low cost alternative could be the plant based virus like particles (VLPs) used against H1N1 influenza. Medicago Inc. has described the production of VLPs in plants (Rosales-Mendoza et al., 2020). The specific proteins can be expressed into the desired plant with the help of agrobacterium mediated transformation and can be grown in the desired location. Antigens are released as bioencapsulated vaccine from the ingested recombinant plant which later on get absorbed by M cells in the intestine and pass to the macrophages and antigen presenting cells thereby activating immune system and induce production of specific antibodies and memory T cells against the antigen (Sartaj Sohrab et al., 2017). So far many of the plant based vaccine candidates have been tested and entered into clinical trials like the vaccine against swine flu, hepatitis B (Govea-Alonso et al., 2014). As per the news articles, Medicago Inc. has started Phase I trial of its plant derived COVID 19 vaccine named, CoVLP following the single adjuvanted mediated positive immunogenic effects obtained in the preclinical testing. According to the enterprise, VLPs have a multi-modal mechanism of action that works differently to inactivated vaccines, by activating both antibody and cell-mediated responses. The adjuvants that will be used alongside the CoVLP vaccine candidate are GlaxoSmithKline's pandemic adjuvant technology and Dynanvax's CpG 1018 (Balfour, 2020).
6. Natural products as immunomodulators
Natural products mainly comprising of Ayurvedic medicines, and Traditional Chinese medicines (TCM) are used for decades as the regulator of the immune system. These compounds either alone or in combinations are used for centuries in both Ayurveda and alternative medicine. Studies have documented their role in the treatment or prevention of many viral infections including influenza, HIV, SARS, and MERS (Gyawali et al., 2020; Islam et al., 2020; Leung, 2007). These medicines boost the immune system and also participates in the innate and adaptive immune regulatory pathways (Wang et al., 2018). The use of natural products and TCM to prevent or treat COVID-19 is largely inspired by the treatment of SARS. Many trials are registered in the clinicaltrials.gov for investigating the role of traditional Chinese medicines and natural products (Nigella sativa, natural honey, curcumin, Boswellia, and vitamin C) in COVID-19 patients (National Library of Medicine, 2020). Compounds like Withania somnifera (Ashwagandha), Tinospora cordifolia (Guduchi), Asparagus racemosus (Shatavari), Phylanthus embelica (Amalaki), and Glyceriza glabra (Yashtimadhu) possess the potential immunomodulatory activity and hence they may be considered for prophylaxis and as an add-on treatment for COVID-19 (Balasubramani et al., 2011; Tillu et al., 2020). Rajkumar et al. have highlighted the possible role of Ayurveda in the treatment of COVID-19 through psychoneuroimmune pathways (Rajkumar, 2020). Apart from Ayurveda, medicines from traditional Siddha system has been found effective as immune system booster in COVID-19 patients. Kabasura kudineer, a herbal concoction regarded as immune booster and capable of combating viral infections found to be helpful in regaining the lost sense of smell in COVID 19 patients. Currently a clinical trial is ongoing on Kabasura kudineer and vitamin C-zinc supplementation in the management of mild COVID 19 patient (CTRI/2020/05/025215, 2020; Livemint, 2020; Soruban and Rajeev, 2020). TCM like Chinese Rhubarb extracts, Houttuynia cordata extract, hesperetin, Fructus Forsythiae, etc. have also demonstrated anti-inflammatory activity by inhibiting the cytokines like TNF-α, IL-1β, and IL-6 and anti-viral activity by inhibition of 3CLpro and RdRp protein (Yang et al., 2020a, Yang et al., 2020b). Recently, a systematic survey by Lee et al. has reported the beneficial role of TCM in COVID-19 patients (Lee et al., 2020). TCM has been used as a treatment of COVID-19 in China, and Qingfei Paidu decoction has reported treating 214 confirmed cases. Yang et al. have reported that the rate of TCM treatment of COVID-19 in China was 87% and only 5% of patients have worsened clinical manifestations (Yang et al., 2020a, Yang et al., 2020b). To date, more than 50 clinical trials have registered in China for the evaluation of TCM in COVID-19. Although shreds of evidence are available for the efficacy of TCM and natural products in COVID-19 patients, still the randomized clinical trials in larger populations are required to evaluate their efficacy and safety in COVID-19 patients.
7. Current status of vaccine candidates in pipeline
Currently, as of 29th October [2020] there are 201 vaccine candidates active globally, of which 45 are in clinical phase and 156 in preclinical phase. List of vaccine candidates under different platforms in different evaluation stages summarized in Table 4. The time required by these vaccine candidates to become available in the market depends on the success of all the phases of clinical trials. As discussed further in this section, 10 of the vaccine candidates have successfully passed phase 1 and phase 2 trials and found to have generated anti-SARS-CoV-2 immune responses and are safe to use, however phase 3 trials for the same are ongoing.
7.1. ChAdOx1 nCoV-19 vaccine
ChAdOx1 nCoV-19 vaccine (AZD1222) consists of the non replicating simian adenovirus vector ChAdOx1 containing the full-length structural spike protein of SARS-CoV-2 developed by University of Oxford and AstraZeneca. The preliminary report released by the research group shows that the candidate ChAdOx1 nCoV-19 vaccine given at a dose of 5 × 101⁰ viral particles was safe and tolerated, however a higher reactogenicity profile was observed than the control vaccine, meningococcal conjugate vaccine MenACWY. The reactogenicity was reduced on administration of 1 g paracetamol for the first 24 h after vaccination. 14 days after the first dose of vaccine the cellular responses were observed and on day 28 post vaccination the humoral responses to spike protein, i.e. the spike specific antibodies were found to be at peak. The second dose of vaccine administered after 28 days of first dose induced neutralizing antibodies in all the participants and the reactogenicity was also found to be reduced after the second dose. Overall, the two doses of vaccine was found to be sufficient to induce potent cellular and humoral immunogenicity in all the participants and no serious adverse reactions occurred. The study was conducted in the healthy individuals excluding all the potential participants with recent COVID-19-like symptoms or with a history of positive PCR test for SARS-CoV-2, however a small number of participants found to have high levels of neutralizing antibody at baseline which probably indicates prior asymptomatic infection (Folegatti et al., 2020).
In conclusion, ChAdOx1 nCoV-19 showed an acceptable safety profile, and sufficient immunogenicity and reduced reactogenecity when given with paracetamol. A single dose found to elicit both humoral and cellular responses against SARS-CoV-2 and booster dose required to enhance neutralizing antibody concentration. The preliminary results supported the large scale evaluation of vaccine in an ongoing phase 3 programme.
7.2. mRNA-1273 vaccine
The mRNA-1273 vaccine is a lipid nanoparticle encapsulated, nucleoside-modified messenger RNA (mRNA)-based vaccine developed by Moderna and NIAID. It encodes the spike glycoprotein (S-protein) present on the surface of SARS-CoV-2. The preliminary findings reported that it generate sufficient immunogenic response and induce vigorous binding antibody responses to both full-length spike protein and receptor-binding domain in all participants after the first vaccination. The immune responses were found to increase with time and increasing dose of the vaccine i.e. 25-μg, 100-μg and 250-μg. And high neutralizing antibody responses were elicited in a dose-dependent fashion. The two-dose vaccination schedule was finalized because of its low pseudovirus neutralizing activity even after the 2 weeks of first vaccination. No serious toxicity was reported after the two dose series of vaccination; systemic adverse events after the first vaccination reported were all graded mild to moderate in severity. Of the three doses evaluated 250 μg, 100 μg and 50 μg, the 100 μg dose elicits high neutralization responses and more favorable reactogenicity profile than that of the higher dose. The rapid and vigorous immunogenicity profile of the candidate vaccine is attributed to the presentation of naturally folded conformation of the S protein designed as antigen coupled with lipid nanoparticle delivery system and using modified mRNA to avoid early inactivation of interferon associated genes. The current report released by research group comprised the result of follow-up of participants through day 57, hence the durability of the immune responses and their characterization is not yet established. A phase 2 trial of mRNA-1273 in 600 healthy adults, evaluating doses of 50 μg and 100 μg is ongoing (ClinicalTrials.gov number, NCT04405076) and the phase 3 trial to evaluate 100 μg dose is expected to begin soon (Jackson et al., 2020).
7.3. Ad5-vectored COVID-19 vaccine
The candidate vaccine is a type of non replicating adenovirus type Ad5-vectored COVID-19 vaccine developed by CanSino Biological Inc. and Beijing Institute of Biotechnology. It is found to elicit both cellular and humoral response in 95% of the participants by the day 28 post vaccination in both doses, i.e. 1 × 1011 viral particles and 5 × 101⁰ viral particles. No serious adverse reactions were reported, those reported were all ranging from mild to moderate in severity and resolved within a short period of time maximum by 48 h of reaction onset. Earlier three doses were given to the participants, but higher proportions of grade 3 adverse reactions were reported in the higher dose of 1.5 × 1011 viral particles compared with the low dose (5 × 1010 viral particles) and middle dose (1 × 1011 viral particles). Further investigation revealed that the vaccine at 5 × 1010 viral particles had a better safety profile than the vaccine at 1 × 1011 viral particles and also shows the better immunogenicity profile. The preliminary report suggests the acceptable safety profile of Ad5-vectored COVID-19 vaccine. Apart from the results, the major obstacle that the researchers face with this vaccine candidate was the high pre-existing anti-Ad5 immunity in the participants with older age (55 years or older) due to which single dose of vaccine was not inducing high levels of humoral immune responses. Therefore, researchers proposed to use the additional dose of vaccine in the older age population to gain the required effects. They will be evaluating it in phase 2b stages of the ongoing trial. Moreover rest of the population showed better results (in terms of safety profile and adequate immunogenicity) with the single immunization of Ad-5 vectored COVID-19 vaccine at dose 5 × 1010 viral particles making it a potential candidate for emergency vaccination (Zhu et al., 2020). In conclusion, the results of the trial have shown acceptable results supporting the testing of candidate vaccine at dose 5 × 1010 viral particles further in phase 2b and phase 3 trial in healthy adults.
7.4. Inactivated COVID-19 vaccine
The candidate vaccine is an inactivated whole SARS-CoV-2 virus vaccine developed by the Wuhan Institute of Biological Products and Sinopharm. The whole virus pathogen was cultivated in vitro in cell lines and the infected cells further inactivated twice using β-propiolactone under specific conditions and further adsorbed to 0.5 mg alum. The phase 1 trial was conducted using three doses with 2.5 μg, 5 μg and 10 μg antigen protein content per dose and the results showed the inactivated vaccine candidate to have better safety profiles comparable to the other vaccines under different platforms and the neutralizing antibody titres induced were also adequate in all the three doses. No serious adverse event was reported, only mild to moderate self limiting reactions were reported and the incidence rate of adverse reactions associated with the current vaccine candidate was very low nearly 15% as compared to the other vaccines where the incidence rate is reported around 60% to even 100% in some studies. Phase 2 trial was conducted using a middle dose of vaccine, i.e. 5 μg antigen protein content and the results showed the vaccine candidate is able to effectively produce antibody titer with minimal adverse reactions reported. However researchers came to conclusion that booster dose is required to generate adequate immunogenic response, also the Phase 1 and Phase 2 trial indicated that with longer interval (21 days and 28 dya) between the first dose and subsequent booster dose generates higher antibody titers as compared to shorter interval group (14 day schedule) (Zhu et al., 2020). In conclusion, the interim analysis of the inactivated vaccine showed the acceptable safety and better immunogenic profile of the candidate vaccine supporting its longer term adverse events assessment in ongoing phase 3 trials.
7.5. BNT162b1 RNA vaccine
The candidate vaccine is the lipid nanoparticle encapsulated mRNA based SARS-CoV-2 vaccine named as BNT162b1 developed by BioNTech, Fosun Pharma and Pfizer. It consists of the nucleoside modified messenger RNA that encodes the receptor binding domain of the SARS-CoV-2 spike protein. Researchers have administered the BNT162b1 vaccine candidate at three different doses 10 μg, 30 μg and 100 μg and found that the vaccine candidate exhibit acceptable safety profile and elicited adequate antibody titer. Based on the tolerability profile, the participants receiving two of the doses 10 μg and 30 μg was given second booster dose and found to show sharper dose vs immunogenic responses while no data for immunogenic response was reported for the dose 100 μg till now since no second vaccination was provided to the participants to avoid the adverse reactions and the study is ongoing. Results indicated higher reactogenicity after the booster dose, however symptoms were limited to mild to moderate in severity which were resolved within few days of onset (Zhu et al., 2020). In conclusion, the RNA based vaccine candidate BNT162b1 showed acceptable safety profile and also found to produce adequate antibody titre after booster dose, hence it supports the long term assessment of the candidate vaccine for efficacy and tolerability in the ongoing phase 3 trials.
7.6. Gamaleya's Sputnik V
It is the type of non replicating adenovirus (rAd26-S + rAd5-S) type vaccine developed by Gamaleya Research Institute Epidemiology and Microbiology, Health Ministry of the Russian Federation. The vaccine consists of the two components, one with the recombinant adenovirus vector based on the human adenovirus type 26 and another with the adenovirus vector based on the human adenovirus type 5, both containing SARS-CoV-2 S protein gene. This adenovirus vector-based vaccine was registered by the Russian Ministry of Health on August 11 and became the first registered COVID-19 vaccine on the market. Prior to its registration and market launch, the vaccine had successfully cleared preclinical trial on various animals and also its safety and efficacy was assessed in humans in Phase 1 and 2 clinical trials. The first component, adenovirus vectored type 26 vaccine containing SARS-CoV-2 S protein gene was given as the first vaccination dose and another component, adenovirus vectored type 5 vaccine containing SARS-CoV-2 S protein gene was given as second vaccination dose to all the participants. No unwanted side effects were reported and the vaccine induced strong antibody and cellular immune response. Currently, the post registration Phase 3 trial is ongoing involving more than 40000 participants in Russia and Belarus and the plan to execute the Phase 3 trial of Sputnik V in various other countries like UAE, India, Venezuela, Egypt and Brazil is underway (Sputnik V, 2020).
7.7. Bharat Biotech's Covaxin
It is the India's first vaccine against COVID-19 and is an inactivated whole SARS-CoV-2 virus vaccine developed by Bharat BioTech, Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV). It has successfully cleared Phase I/II human trial and is given permission for Phase III trial by DCGI (Drug Controller General of India). The vaccine trial Phase I/II has been conducted in 12 hospitals in different cities across country, in healthy volunteers with no co-morbidity conditions. The vaccine was found to generate robust immune responses, thereby preventing infection and disease in the primates upon high amount of exposure to live SARS-CoV-2 virus. As per the vaccine makers the phase III study would cover around 28,500 subjects aged 18 years and above, however the complete safety and immunogenicity data of the phase II trial is still not revealed (Ray, 2020; Sharad Sharma, 2020).
7.8. Cadila's ZyCoV-D vaccine
It is India's second vaccine, a type of DNA plasmid vaccine expressing SARS-CoV-2 S protein developed by Zydus Cadila Healthcare. Currently, the phase II trial of the candidate vaccine is underway. The Phase I trial of vaccine was conducted on healthy volunteers and was found safe in all the participants (Ray, 2020; CTRI/2020/07/026352, 2020).
8. Concluding remarks
COVID-19 pandemic has imposed a huge financial and social burden to the world. With regard to its continuously evolving nature, it remains a challenging task to restrict the transmission of infection among the population. One of the prominent approaches for preventing SARS-CoV-2 infection is the development of vaccines against COVID-19. A great deal of work has done so far and till now 33 vaccine candidates have entered into a clinical trial and 143 are in the preclinical phase. Despite the extraordinary efforts of the firms and researchers across the globe for developing the COVID-19 vaccines, there is still a lot more to explore that could be helpful in developing more specific vaccine. Gathering of important information like immunization route, finding more target antigen(s) apart from targeting S protein as antigen only, can be helpful in eradicating the infection. The immunopathological basis of COVID-19 need to be explored more so that its immune evasion mechanism can be targeted and it will also provide deeper insights into vaccine designing strategies. The three main criteria which should be taken into consideration while developing a vaccine are: speed, scale up manufacturing and global access. The astonishing efforts by researchers across the globe in terms of scale and speed of vaccine development have fastened the vaccines development journey from bench to bedside within a few months only. The work done so far in this regard is very commendable as 5 of the candidates are already in Phase 3 clinical trial and hopefully will be available by the end of 2020. This represents a huge change in the conventional pathway of vaccine development which usually takes 5–10 years timescale. Along with speeding up the development process, it is equally important to evaluate the effectiveness and safety of vaccine at each step, and this has been the major hurdle for researchers in establishing the vaccine's efficacy so far. However, the phase 3 trial will play a major role in establishing the vaccine that is safe and effective in a large and diverse population. Finally, the animal models specific for COVID-19 should be established in order to assess vaccine efficacy. Also, the regulatory authorities should need to ensure the equal distribution of vaccines to all the affected areas.
Declaration of competing interest
The authors declare no conflict of interest.
References
- Adnan M., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin , transmission , and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ahmed S.F., Quadeer A., McKay M. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:1–15. doi: 10.3390/v12030254. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- An J. 2020. Clinical Characteristics of the Recovered COVID-19 Patients with Re-detectable Positive RNA Test. medRxiv. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Balasubramani S.P., Venkatasubramanian P., Kukkupuni S.K., Patwardhan B. Plant-based Rasayana drugs from Ayurveda. Chin. J. Integr. Med. 2011;17:88–94. doi: 10.1007/s11655-011-0659-5. [PubMed] [CrossRef] [Google Scholar]
- Balfour H. 2020. Plant-based COVID-19 Vaccine Enters Phase I Trials.https://www.europeanpharmaceuticalreview.com/news/124092/plant-based-covid-19-vaccine-enters-phase-i-trials/ [WWW Document] accessed 10.27.20. [Google Scholar]
- Barría M.I. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J. Infect. Dis. 2013;207:115–124. doi: 10.1093/infdis/jis641. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Becker R.C. Vol. 50. 2020. pp. 499–511. (COVID-19-associated vasculitis and vasculopathy). [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Beyrouti R. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Bian H., Zheng Z.H., Wei D., Zhang . 2020. Meplazumab Treats COVID-19 Pneumonia: an Open-labelled, Concurrent Controlled Add-on Clinical Trial. medRxiv. [CrossRef] [Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cao B. A trial of lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;1–13 doi: 10.1056/NEJMoa2001282. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Carvalho A. SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: implications for detection and transmission of COVID-19 disease. Am. J. Gastroenterol. 2020;115:942–946. doi: 10.14309/ajg.0000000000000667. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cheng Y. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cochrane . 2020. Cochrane COVID-19 Study Register.https://covid-19.cochrane.org/ [WWW Document] accessed 10.29.20. [Google Scholar]
- CTRI/2020/05/025215 . 2020. CTRI/2020/05/025215.http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=43769&EncHid=&userName=A prospective, single centre, randomized open labelled comparative clinical study to evaluate [WWW Document] accessed 10.25.20. [Google Scholar]
- CTRI/2020/07/026352 . 2020. CTRI/2020/07/026352.http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45306&EncHid=&userName=vaccine [WWW Document] accessed 10.25.20. [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Dandekar A.A., Perlman S. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Deshmukh H.S. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 2014;20:524–530. doi: 10.1038/nm.3542. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- D'Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin. Gastroenterol. Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Fan Y.Y. Characterization of SARS-CoV-specific memory T cells from recovered individuals 4 years after infection. Arch. Virol. 2009;154:1093–1099. doi: 10.1007/s00705-009-0409-6. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Folegatti P.M. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. Elsevier. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/BST.2020.01047. [PubMed] [CrossRef] [Google Scholar]
- García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Gautret P., Lagier J., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open- label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Geiben-Lynn R., Greenland J.R., Frimpong-Boateng K., Letvin N.L. Kinetics of recombinant adenovirus type 5, vaccinia virus, modified vaccinia ankara virus, and DNA antigen expression in vivo and the induction of memory T-lymphocyte responses. Clin. Vaccine Immunol. 2008;15:691–696. doi: 10.1128/CVI.00418-07. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Govea-Alonso D.O., Cardineau G.A., Rosales-Mendoza S. In: Principles of Plant-Based Vaccines BT - Genetically Engineered Plants as a Source of Vaccines against Wide Spread Diseases: an Integrated View. Rosales-Mendoza S., editor. Springer New York; New York, NY: 2014. pp. 1–14. [CrossRef] [Google Scholar]
- Gyawali R. A review on ayurvedic medicinal herbs as remedial perspective for COVID-19. J. Karnali Acad. Heal. Sci. 2020;3:1–21. doi: 10.3126/jkahs.v3i0.29116. [CrossRef] [Google Scholar]
- He Y., Rappuoli R., De Groot A.S., Chen R.T. Emerging vaccine informatics. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/218590. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hekele A. Rapidly produced SAM ® vaccine against H7N9 influenza is immunogenic in mice. Emerg. Microb. Infect. 2013;2:1–7. doi: 10.1038/emi.2013.54. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hemann E.A., Kang S.M., Legge K.L. Protective CD8 T cell mediated immunity against influenza A virus infection following influenza virus-like particle vaccination1. J. Immunol. 2013;191:2486–2494. doi: 10.1038/jid.2014.371. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Holshue M.L. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020 doi: 10.1038/s41579-020-00459-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hur S. Double-stranded RNA sensors and modulators in innate immunity. Annu. Rev. Immunol. 2019;37:349–375. doi: 10.1146/annurev-immunol-042718-041356. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Islam M.T. Natural products and their derivatives against coronavirus: a review of the non‐clinical and pre‐clinical data. Phyther. Res. 2020;34:2471–2492. doi: 10.1002/ptr.6700. [PubMed] [CrossRef] [Google Scholar]
- Jackson L.A. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. Mass Medical Soc. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Katzelnick L.C. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. 358, 929–932. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kim J.H.J.J. DNA vaccines against Influenza viruses. Vaccines Pandemic Influ. 2009;333:197–210. doi: 10.1007/978-3-540-92165-3. [PubMed] [CrossRef] [Google Scholar]
- Kumar A., Meldgaard T.S., Bertholet S. Novel platforms for the development of a universal influenza vaccine. Front. Immunol. 2018;9:1–14. doi: 10.3389/fimmu.2018.00600. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lee D.Y.W., Li Q.Y., Liu J., Efferth T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: clinical experience and scientific basis. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153337. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Leng Z. Transplantation of ACE2- Mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Leung P.-C. The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis. Am. J. Chin. Med. 2007;35:575–581. doi: 10.1142/S0192415X07005077. [PubMed] [CrossRef] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [PubMed] [CrossRef] [Google Scholar]
- Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus [6] N. Engl. J. Med. 2003;349:508–509. doi: 10.1056/NEJM200307313490520. [PubMed] [CrossRef] [Google Scholar]
- Li D. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study. Signal Transduct. Target. Ther. 2020;5:1–3. doi: 10.1038/s41392-020-0163-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. Elsevier Ltd. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ling W. C-reactive protein levels in the early stage of COVID-19. Med. Maladies Infect. 2020;50:332–334. doi: 10.1016/j.medmal.2020.03.007. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Liu W.J., Zhao M., Liu K., Xu K., Wong G. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antivir. Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Livemint . 2020. Siddha Medicine in COVID-19.https://www.livemint.com/news/india/nearly-6-000-covid-patients-cured-through-siddha-medicine-in-tamil-nadu-11597051467504.html [WWW Document] accessed 10.25.20. [Google Scholar]
- Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:1–3. doi: 10.1016/S0140-6736(20)30251-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- María R.R., Arturo C.J., Alicia J., Paulina M.G., Gerardo A. Vaccines. InTech; Rijeka, Croatia: 2017. The impact of bioinformatics on vaccine design and development. [CrossRef] [Google Scholar]
- Marzi A. Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J. Infect. Dis. 2011;204:S1066–S1074. doi: 10.1093/infdis/jir348. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Moon C. Fighting COVID-19 exhausts T cells. Nat. Rev. Immunol. 2020;20:277. doi: 10.1101/2020.02.18.20024364. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Mubarak A., Alturaiki W., Hemida M.G. Middle East respiratory syndrome coronavirus ( MERS-CoV ): infection , immunological response , and vaccine development. J. Immunol. Res. 2019;2019:1–11. doi: 10.1155/2019/6491738. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Nascimento I.P., Leite L.C.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012;45:1102–1111. doi: 10.1590/S0100-879X2012007500142. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- National Library Of Medicine . 2020. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=vaccines&cntry=&state=&city=&dist=&Search=Search [WWW Document] accessed 10.28.20. [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Prompetchara E., Chutitorn Ketloy T.P. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [PubMed] [CrossRef] [Google Scholar]
- Qin C. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Rajkumar R.P. Ayurveda and COVID-19: where psychoneuroimmunology and the meaning response meet. Brain Behav. Immun. 2020;87:8–9. doi: 10.1016/j.bbi.2020.04.056. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Rappuoli R. Reverse vaccinology. Curr. Opin. Microbiol. 2000;3:445–450. doi: 10.1016/S1369-5274(00)00119-3. [PubMed] [CrossRef] [Google Scholar]
- Ray Anulekha. 2020. COVID-19 Vaccine may be Ready by December References.https://www.livemint.com/science/health/covid-19-vaccine-may-be-ready-by-december-says-govt-a-look-at-india-s-vaccine-journey-11603359897416.html#box_11603359897416 [WWW Document] accessed 10.27.20. [Google Scholar]
- Rodrigues T.S. Inflammasome activation in COVID-19 patients. 2020. medRxiv. [CrossRef]
- Rodríguez-Gascón A., del Pozo-Rodríguez A., Solinís M.Á. Development of nucleic acid vaccines: use of self-amplifying RNA in lipid nanoparticles. Int. J. Nanomed. 2014;9:1833–1843. doi: 10.2147/IJN.S39810. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Roldão A., Mellado M.C.M., Castilho L.R., Carrondo M.J.T., Alves P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [PubMed] [CrossRef] [Google Scholar]
- Rollier C.S., Reyes-Sandoval A., Cottingham M.G., Ewer K., Hill A.V.S. Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol. 2011;23:377–382. doi: 10.1016/j.coi.2011.03.006. [PubMed] [CrossRef] [Google Scholar]
- Roncati L., Nasillo V., Lusenti B., Riva G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann. Hematol. 2020;99:1419–1420. doi: 10.1007/s00277-020-04066-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Rosales-Mendoza S., Márquez-Escobar V.A., González-Ortega O., Nieto-Gómez R., Arévalo-Villalobos J.I. What does plant-based vaccine technology offer to the fight against COVID-19? Vaccines. 2020;8:183. doi: 10.3390/vaccines8020183. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Sartaj Sohrab S., Suhail M., Kamal M.A., Husen A., I Azhar E. Recent development and future prospects of plant-based vaccines. Curr. Drug Metabol. 2017;18:831–841. doi: 10.2174/1389200218666170711121810. [PubMed] [CrossRef] [Google Scholar]
- Seib K.L., Zhao X., Rappuoli R. Developing vaccines in the era of genomics: a decade of reverse vaccinology. Clin. Microbiol. Infect. 2012;18:109–116. doi: 10.1111/j.1469-0691.2012.03939.x. [PubMed] [CrossRef] [Google Scholar]
- Sekaly R.P. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Sharad Sharma D.D.R. 2020. Covaxin, Bharat Biotech's Coronavirus Vaccine, Cleared for Phase 3 Trials.https://www.ndtv.com/india-news/covaxin-bharat-biotechs-coronavirus-vaccine-cleared-for-phase-3-trials-2314348 [WWW Document] accessed 10.24.2020. [Google Scholar]
- Soruban T., Rajeev S.R.P. Comparative study on pandemic crisis - Covid19 and Sri Lankan Siddha system of medicine – a literature review. EJPMR. 2020;7:292–299. [Google Scholar]
- Sputnik V., clinical trials . 2020. Vaccine against COVID-19: Sputnik V.https://sputnikvaccine.com/about-vaccine/clinical-trials/ [WWW Document] accessed 10.20.20. [Google Scholar]
- Spychalski P., Błażyńska-Spychalska A., Kobiela J. Estimating case fatality rates of COVID-19. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30246-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Su H. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Tan L. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 2020;5:1–3. doi: 10.1038/s41392-020-0148-4. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Tang F. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J. Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [PubMed] [CrossRef] [Google Scholar]
- Tillu G., Chaturvedi S., Chopra A., Patwardhan B. Public health approach of Ayurveda and Yoga for COVID-19 prophylaxis. J. Alternative Compl. Med. 2020;26:360–364. doi: 10.1089/acm.2020.0129. [PubMed] [CrossRef] [Google Scholar]
- Ulrich H., Pillat M.M., Tárnok A. Dengue Fever, COVID‐19 (SARS‐CoV‐2), and Antibody‐Dependent Enhancement (ADE): A Perspective. Cytom. Part A. Wiley Online Library. 2020;97(7):662–667. doi: 10.1002/cyto.a.24047. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A. Structure, function, and antigenicity of the SARS- CoV-2 spike glycoprotein. Cell. 2020;180:1–12. doi: 10.1016/j.cell.2020.02.058. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel Coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS Coronavirus. J. Virol. 2020;94:1–9. doi: 10.1128/jvi.00127-20. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang K., Conlon M., Ren W., Chen B.B., Bączek T. Natural products as targeted modulators of the immune system. J. Immunol. Res. 2018;2018:1–2. doi: 10.1155/2018/7862782. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- WHO . Who; 2020. Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ [WWW Document] accessed 10.28.20. [Google Scholar]
- World Health Organization . 2020. Draft Landscape of COVID 19 Candidate Vaccines.https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines [WWW Document] accessed 10.30.20. [Google Scholar]
- Wu A. Genome composition and divergence of the novel Coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wu C. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wu F. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Xing Y. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveill. 2020:1–5. doi: 10.2807/1560-7917.ES.2020.25.10.2000191. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Yang L. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020;5:1–8. doi: 10.1038/s41392-020-00243-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Yang, Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Yuan J. 2020. Clinical Characteristics on 25 Discharged Patients with COVID-19 Virus Returning. medRxiv. [CrossRef] [Google Scholar]
- Zhang J., Ma K., Li H., Liao M., Qi W. The continuous evolution and dissemination of 2019 novel human Coronavirus. J. Infect. 2020;20:1–5. doi: 10.1016/j.jinf.2020.02.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. 2020. Pangolin Homology Associated with 2019-nCoV. bioRxiv. [CrossRef] [Google Scholar]
- Zhao Q., Li S., Yu H., Xia N., Modis Y. Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol. 2013;31:654–663. doi: 10.1016/j.tibtech.2013.09.002. [PubMed] [CrossRef] [Google Scholar]
- Zhao Jincun. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. U.S.A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhou P. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhou Y. Cold Spring Harbor Laboratory; 2020. Aberrant Pathogenic GM-CSF+ T Cells and Inflammatory CD14+ CD16+ Monocytes in Severe Pulmonary Syndrome Patients of a New Coronavirus. BioRxiv. [CrossRef] [Google Scholar]
- Zhu F.-C. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1038/s41586-020-2639-4. Elsevier. [PMC free article] [PubMed] [CrossRef] [Google Scholar]